MALVERN PEAQ Isothermal Titration Calorimeter
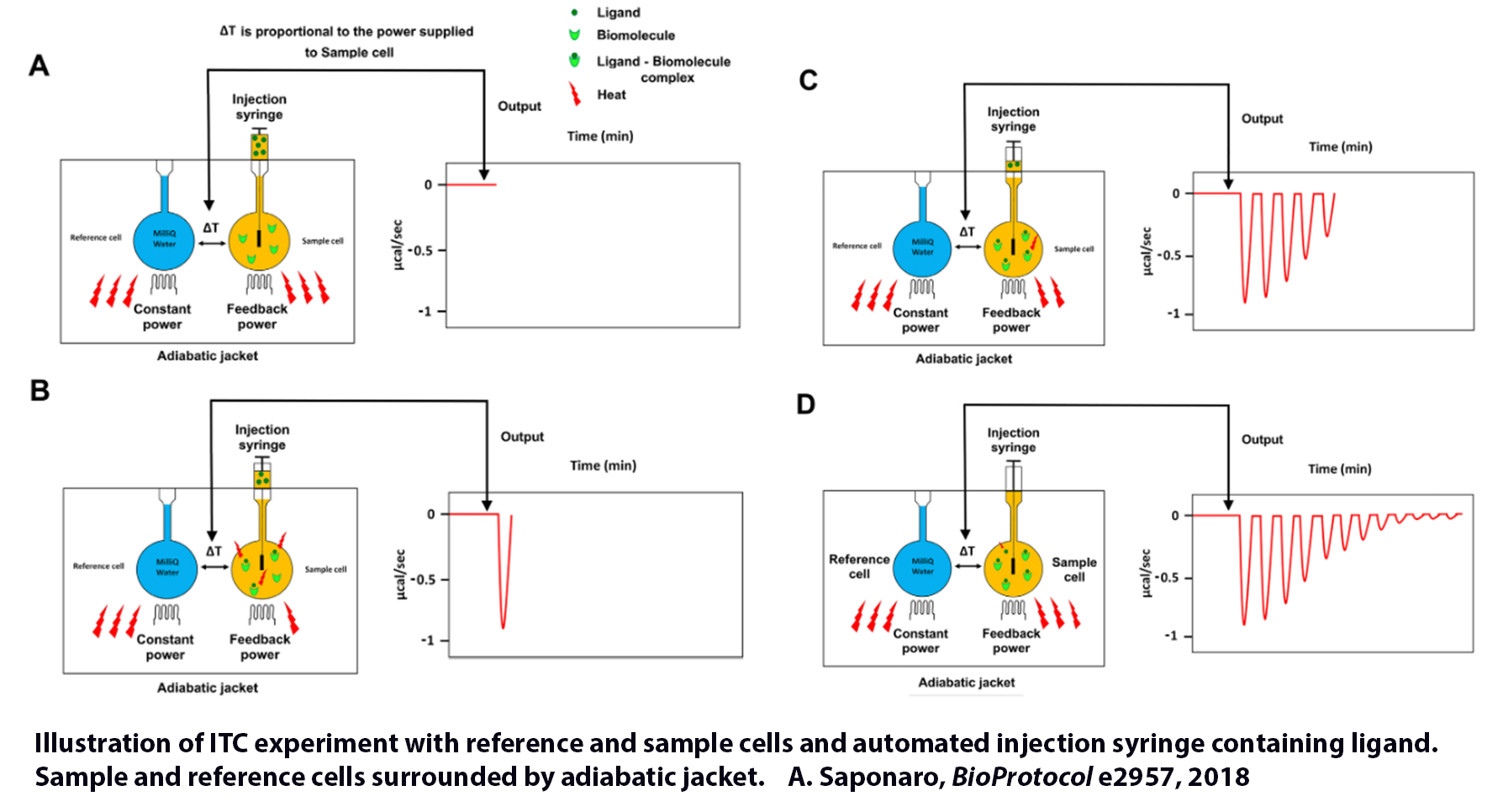
The PEAQ-ITC Unit measures heat evolved or absorbed in solution state samples due to mixing precise amounts of interacting molecules such as a protein and a ligand. A pair of identical coin-shaped cells (the sample and reference cells) are enclosed in an adiabatic chamber. The sample and reference cells are accessible for filling and cleaning through the top of the unit. The sample cell is on the right as one faces the front of the unit. Access items travel from the top exterior of the instrument to the cells. Both cells and the access stems must be filled with liquid completely during operation. This requires approximately 0.5 mL per cell, even though the working volume of the cell is only 0.35 mL. A spinning syringe injects and mixes the interacting species, and spin rates are user-selected. The normal operating range is 2 °C to 80°C.
In the typical experiment, temperature differences between the reference cell and the sample cell are measured, calibrated to power units, displayed to the user, and saved to disk. The data channel is called the DP signal, or the differential power between the reference and sample cells This signal is sometimes referred to as the "feedback" power used to maintain temperature equilibrium. Calibration of this signal is obtained electrically by administering a known quantity of power through a resistive heater element located on the cell.
For the measurements, the syringe containing the "ligand" is titrated (injected) into the cell containing a solution of the "macromolecule." An injection that results in the evolution of heat (exothermic) within the sample cell causes a negative change in the DP power since the heat evolved chemically provides heat that the DP feedback is no longer required to provide. The opposite is true for endothermic reactions. Since the DP has power units, the peak's time integral yields a thermal energy measurement, DH. This heat is released or absorbed in direct proportion to the amount of binding that occurs. When the macromolecule in the cell becomes saturated with added ligand, the heat signal diminishes until only the background heat of dilution is observed.
With the PEAQ-ITC system, the experiment is computer-controlled. The user inputs the experimental parameters (temperature, number of injections, injection volumes), and the computer carries out the experiment. Origin software is then used to analyze the ITC data using fitting models to calculate reaction stoichiometry (n), binding constant (Kb), enthalpy (DH), and entropy (DS).
From PEAQ-ITC User's Manual, Malvern, LLC
Jelesarov, I. and Bosshard, H. R. (1999) Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition, J. Mol. Recognit,, 12:3-18.
Biocalorimetry: Applications of Calorimetry in the Biological Sciences, ed. John E. Ladbury and Babur Z. Chowdhry, 1998. John Wiley & Sons.